New Liquid Crystal-Based Technology Rapidly Detects Multiple Pathogens in Food
Posted Oct. 26, 2011Crystal Diagnostics unveils unique food-safety testing system
Each year, food-borne pathogens cause an estimated 48 million illnesses (affecting one in six people), 128,000 hospitalizations and 3,000 deaths, according to the Centers for Disease Control and Prevention. Variant E. coli strains in ground beef and lettuce, Salmonella in turkey and chicken, and Listeria in cantaloupe and romaine lettuce have sparked widespread recalls due to contamination.
 The need for faster detection of harmful pathogens in food is evident. A rapid, reliable method that can test for multiple pathogens simultaneously gives food-safety assurance to farmers, food processors, distributors, retailers and ultimately to the public. The Crystal Diagnostics MultiPath SystemTM addresses this need. The novel technology, in development since 2006, uses liquid crystals to detect multiple harmful pathogens in food in a single test, offering significant time savings over the most common testing methods.
The need for faster detection of harmful pathogens in food is evident. A rapid, reliable method that can test for multiple pathogens simultaneously gives food-safety assurance to farmers, food processors, distributors, retailers and ultimately to the public. The Crystal Diagnostics MultiPath SystemTM addresses this need. The novel technology, in development since 2006, uses liquid crystals to detect multiple harmful pathogens in food in a single test, offering significant time savings over the most common testing methods.
Crystal Diagnostics introduced this new technology with a demonstration today at the company’s manufacturing facility in the Kent State Centennial Research Park located at 1950 State Route 59 in Kent, Ohio. Crystal Diagnostics is the exclusive licensee of fundamental liquid crystal biosensor technologies developed jointly by a research partnership between Kent State University and Northeast Ohio Medical University, previously known as the Northeastern Ohio Universities Colleges of Medicine and Pharmacy.
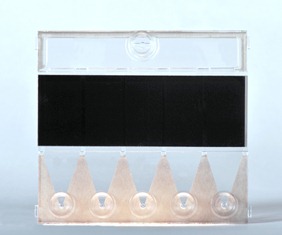
Crystal Diagnostics’ technology includes two pieces of equipment. The first is a “cassette” containing five individual “cells,” two of which are control cells and three are test cells. A prepared (enriched) sample — from ground beef or lettuce, for example — is mixed with liquid crystals and an antibody or antibody “cocktail” for the pathogen or pathogens being sought. The second piece is a “reader.” The cassette is inserted into the reader. If the pathogen or pathogens being sought are present, the liquid crystal — aligned in the reader — will be disrupted. The reader recognizes this disruption and displays the result on an iPad or other device in less than 30 minutes.
In addition to its speed, Crystal Diagnostics’ technology offers other significant advantages:
- A single test can detect multiple pathogens, for example, E. coli O157:H7 plus the other “Big Six” E. coli strains and the strain that appeared in Germany in early 2011. This capability is particularly relevant because the Food Safety and Inspection Service has announced it will require testing for E. coli strains other than O157:H7 beginning next year in beef trim used in ground beef
- In addition to these strains of E. coli, by utilizing the three cells in the cassette independently, the test can detect Salmonella, and another pathogen, such as Listeria, in the same test.
- The nature of the technology very significantly reduces false positives and negatives even before the built-in protection of the two control cells is considered. False positives require longer product holds while retesting is accomplished; false negatives represent a different and a vastly more serious problem for food producers.
“The need for faster yet highly reliable processes to detect pathogens has never been higher given the recent deadly food outbreaks,” said Paul Repetto, CEO of Crystal Diagnostics. “In 2011, a new strain of E. coli appeared in Germany sickening thousands and killing some. Millions of pounds of meat have been recalled at great expense, and most recently 25 people died from Listeria contaminated melons. This new technology can have a profound impact on public health.”
This fall, the equipment will be further beta-tested in the field with leading food processing companies and laboratories.
# # #
About Crystal Diagnostics: Founded in 2006, Crystal Diagnostics (www.crystaldiagnostics.com) is the exclusive licensee of fundamental liquid crystal biosensor technologies developed through a research partnership between Kent State University and Northeast Ohio Medical University. The company has facilities in Kent, Ohio, and Broomfield, Colo.
About NEOMED: Northeast Ohio Medical University (www.neomed.edu), formerly Northeastern Ohio Universities Colleges of Medicine and Pharmacy, is a public medical university and research institution whose mission is to improve the quality of health care in northeast Ohio. NEOMED offers a doctor of medicine (M.D.) and a doctor of pharmacy (Pharm.D.) degree.
About Kent State University: With eight campuses and more than 42,000 students, Kent State University (www.kent.edu) is the second-largest university in Ohio. Kent State is ranked as one of the world’s top universities by Times Higher Education of London and one of the best national universities by U.S. News & World Report. The university also is ranked among the nation’s 77 public research universities by the Carnegie Foundation for the Advancement of Teaching.
Photo Caption:
The Crystal Diagnostics MultiPath SystemTM uses liquid crystals to detect multiple harmful pathogens in food in a single test, offering significant time savings over the most common testing methods. Pictured is the “cassette” used with this new technology.
Media Contacts:
Sylvia R. Tawse, Fresh Ideas Group for Crystal Diagnostics, sylvia@freshideasgroup.com, 303-913-9650
Emily Vincent, Kent State University, evincen2@kent.edu, 330-672-8595
Cristine Boyd, NEOMED, cboyd@neomed.edu, 330-325-6673

Facebook
Twitter
Google+
LinkedIn
Instagram
YouTube
More Ways to Connect